NbN3 Взлом 3.9.1 + Чит Коды
Разработчик: European College of Neuropsychopharmacology (ECNP)
Категория: Медицина
Цена: Бесплатно
Версия: 3.9.1
ID: com.inmanage.iNbNomenclature
Скриншоты

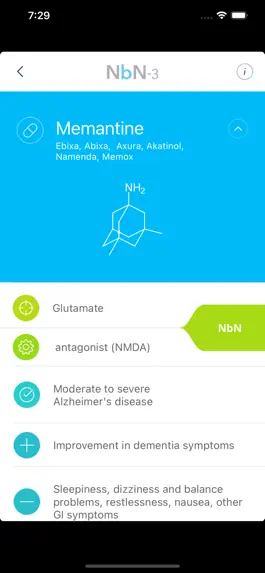
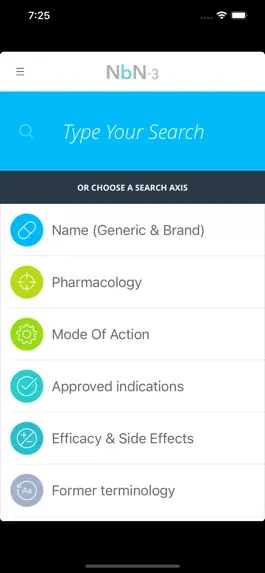
Описание
It has become clear that the current pharmacological nomenclature of psychotropic medications does not reflect our contemporary knowledge, nor does it inform properly the clinician of neuroscience-based prescriptions. Very often we prescribe “antidepressants” for “anxiety” disorders or “second-generation antipsychotics” to depressed patients.
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided five years ago to establish a taskforce and gave it the mission to embed our current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In this second edition 22 new medications were added so NbN includes now 130 medications.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/ molecule/system being modified and the mode/mechanism of action.
We also added 4 additional dimensions
4 Additional Dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Neurobiology – This dimension is focused on the biology. It is divided into preclinical and clinical sections, with the emphasis on the latter
For those who would like to know more abo derived from empirical data.ut the pharmacology, there is a direct link to the relevant site of IUPHAR – our collaborator in this endeavour.
As this is on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertaken.
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided five years ago to establish a taskforce and gave it the mission to embed our current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In this second edition 22 new medications were added so NbN includes now 130 medications.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/ molecule/system being modified and the mode/mechanism of action.
We also added 4 additional dimensions
4 Additional Dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Neurobiology – This dimension is focused on the biology. It is divided into preclinical and clinical sections, with the emphasis on the latter
For those who would like to know more abo derived from empirical data.ut the pharmacology, there is a direct link to the relevant site of IUPHAR – our collaborator in this endeavour.
As this is on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertaken.
История обновлений
3.9.1
2023-07-06
- Bug fixes.
3.9
2023-05-23
- Bug fixes.
3.8
2023-05-09
- Bug fixes.
3.7
2023-05-05
- Bug fixes.
3.6
2023-02-25
- Bug fixes.
3.5
2023-02-24
It has become clear that the current pharmacological nomenclature of psychotropic medications does not reflect our contemporary knowledge, nor does it properly inform the clinician of neuroscience-based prescriptions. Very often we prescribe “antidepressants” for “anxiety” disorders or “second-generation antipsychotics” to depressed patients.
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
3.4
2022-01-11
It has become clear that the current pharmacological nomenclature of psychotropic medications does not reflect our contemporary knowledge, nor does it properly inform the clinician of neuroscience-based prescriptions. Very often we prescribe “antidepressants” for “anxiety” disorders or “second-generation antipsychotics” to depressed patients.
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
3.3
2021-11-24
It has become clear that the current pharmacological nomenclature of psychotropic medications does not reflect our contemporary knowledge, nor does it properly inform the clinician of neuroscience-based prescriptions. Very often we prescribe “antidepressants” for “anxiety” disorders or “second-generation antipsychotics” to depressed patients.
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
This practice is confusing.
• Five international organizations ECNP, ACNP, AsCNP, CINP & IUPHAR decided to establish a taskforce and gave it the mission to embed current neuroscience advances in the nomenclature.
• The scope is to include all the medications with CNS indications and to harness this new nomenclature to help clinicians when they are trying to figure out what would be the next rational “neuropsychopharmacological step”.
• In the third edition, 17 new medications were added so that NbN now includes 147 medications. We also introduced the concept of DDDPs – Different Doses Different Pharmacology (see for example - Doxepin). Additionally, we updated relevant data, especially regarding neurobiology.
This proposed nomenclature aims to reflect the current pharmacological knowledge base and cannot necessarily represent the ultimate scientific truth.
The taskforce that assembled could have taken the stand that our current knowledge base is not enough to define the primary target or the correct mechanisms of action. But as a taskforce, we feel that it’s better to present a cutting-edge scientific interpretation than to wait for the definitive conclusion. We need to treat our patients now, and we cannot postpone treatment until all the facts are known.
Therefore this nomenclature is based on:
1. The need to treat now.
2. Updated neuroscience insights.
3. The judgment of the members of the taskforce.
Along these lines, we have come up with the following proposal:
The Nomenclature:
Pharmacology and Mode of Action – reflects the current knowledge and understanding about the targeted neurotransmitter/molecule/system being modified and the mode/mechanism of action.
We also added 5 dimensions:
Approved Indications – based on the recommendations of major regulatory bodies (e.g. FDA, EMA, etc.)
Efficacy and Side Effects – Driven from positive single, large, RCT and/or “heavy solid weight” clinical data. Only prevalent or life-threatening side effects were included
Practical Note – Summarizes the clinical knowledge that has been "filtered" through the taskforce's "sieve"
Pregnancy – Potential risk of taking the particular medication during pregnancy
Neurobiology – This dimension focuses on biology. It is divided into preclinical and clinical sections, with an emphasis on the latter.
As this is an on-going process, we recognize that the product is imperfect. Based on your feedback (and taking into account the feedback of other colleges) new reports and findings, appropriate updates (e.g. later editions) will be undertake
3.1
2021-08-12
Performance improvements
3.0
2021-08-11
Performance improvements
2.9
2020-11-26
In this version of the app, we fixed some technical issues and added important information and tools such as: a tutorial video of the app., the mission and scope of the NbN, and lists of the 10 pharmacological domains and 9 modes of action used to classify psychotropics.
2.7
2019-08-04
In this new version, we have included new information on doses for each drug. Another change is related to nomenclature; it was simplified, (e.g., instead of talking about “serotonin reuptake inhibitor” we now use “serotonin inhibitor”), yet the extended terminology is brought as the first line in the neurobiology layer. Furthermore we improved and updated the search engine logic for a better and more intuitive user experience.
2.6
2018-08-29
General improvements
2.5
2018-08-26
General improvements
2.4
2018-05-08
General improvements.
2.3
2018-01-09
General improvements
2.2
2017-11-05
IOS 11 bug fixes.
2.1
2017-08-29
General improvements
2.0
2017-05-08
In this updated version we improve and optimize NbN search engine logic by dimensions in order to shorten user search time. Furthermore additional drug were added according to ECNP committee recommendation.
The new and updated version of NbN-2 is an English version, translation to choosen languages will follow.
The new and updated version of NbN-2 is an English version, translation to choosen languages will follow.
1.7.1
2016-05-17
Additional bug fixes
1.7
2016-05-15
Bug fixes
1.6
2016-01-06
Bug fixes
1.5
2015-12-02
This new version of NbN app includes:
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
1.4
2015-09-01
This new version of NbN app includes:
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
1.3
2015-08-20
This new version of NbN app includes:
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
· Updated information
· Faster upload and faster response
· Translation to Japanese and Spanish
· New and improved algorithm
· User friendly interface
All the above will allow better user experience and improve control over the new neuroscience based nomenclature.
Способы взлома NbN3
- Промо коды и коды погашений (Получить коды)
Скачать взломанный APK файл
Скачать NbN3 MOD APK
Запросить взлом
Рейтинг
5 из 5
5 Голосов
Отзывы
D.Uporozzo,
Awesome
In my opinion the relevance and significance of this app just cannot be overestimated 😍
Кузьмина Юля,
Perfect for real-life practice!
Thank you for this app. It’s lovely! 100% of benefit. Bravo!