ODYSSEY Ionic Solids Взлом 1.0.5 + Чит Коды
Разработчик: Wavefunction, Inc
Категория: Образование
Цена: 349,00 ₽ (Скачать бесплатно)
Версия: 1.0.5
ID: com.wavefun.OdyIonicSolids
Скриншоты

Описание
What does the unit cell of sodium chloride look like? How is its structure different from other salts? What relationship, if any, exists between the structures of ionic and metallic solids?
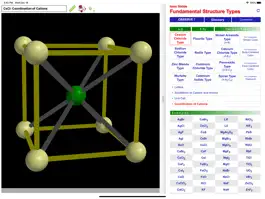
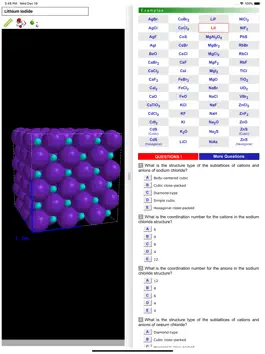
ODYSSEY Ionic Solids is a three-dimensional visualization aid that expands on standard textbook concepts of the structure of ionic crystals. Generic models and concrete examples are provided for twelve structure types: sodium chloride, cesium chloride, zinc blende, wurtzite, fluorite, rutile, nickel arsenide, cadmium chloride, cadmium iodide, calcium chloride, perovskite, and spinel. Unit cells are presented, coordination numbers are highlighted, and the occupancy of cations in the sublattice of anions is illustrated. Models of the cubic structure types for metals (simple cubic, body-centered cubic, and face-centered cubic) are available for comparison. In total, more than a hundred models are available.
All models can be inspected at any orientation and zoom level and with a choice of the drawing radius for the ionic spheres. For any given ion, a “clipping sphere” can be defined that highlights the local surroundings of that ion and hides the remaining structure. Distances between ions can be measured, and a display of ionic charge labels is available. A glossary, comments section, and set of multiple choice questions (with randomized options) are also included.
The structural patterns presented in ionic compounds are not easily appreciated without looking at concrete models. ODYSSEY Ionic Solids helps familiarize learners with the subject and gain the experience needed to explore new structures.
ODYSSEY Ionic Solids is a three-dimensional visualization aid that expands on standard textbook concepts of the structure of ionic crystals. Generic models and concrete examples are provided for twelve structure types: sodium chloride, cesium chloride, zinc blende, wurtzite, fluorite, rutile, nickel arsenide, cadmium chloride, cadmium iodide, calcium chloride, perovskite, and spinel. Unit cells are presented, coordination numbers are highlighted, and the occupancy of cations in the sublattice of anions is illustrated. Models of the cubic structure types for metals (simple cubic, body-centered cubic, and face-centered cubic) are available for comparison. In total, more than a hundred models are available.
All models can be inspected at any orientation and zoom level and with a choice of the drawing radius for the ionic spheres. For any given ion, a “clipping sphere” can be defined that highlights the local surroundings of that ion and hides the remaining structure. Distances between ions can be measured, and a display of ionic charge labels is available. A glossary, comments section, and set of multiple choice questions (with randomized options) are also included.
The structural patterns presented in ionic compounds are not easily appreciated without looking at concrete models. ODYSSEY Ionic Solids helps familiarize learners with the subject and gain the experience needed to explore new structures.
История обновлений
1.0.5
2022-07-23
iOS 15 fixes- minor content updates
1.0.4
2018-12-21
Compatibility fix for certain devices
1.0.3
2016-01-21
- iPad Pro compatibility
- Quarter point penalty for incorrect answers removed
- Additional models
- Quarter point penalty for incorrect answers removed
- Additional models
1.0.2
2014-12-03
- Compatibility fixes for iOS 8.1
- Touch interface atom picking improved
- Touch interface atom picking improved
1.0.1
2014-10-09
- Fix for retina displays under iOS 8
- Improved presentation
- Improved presentation
1.0.0
2014-07-26
Способы взлома ODYSSEY Ionic Solids
- Промо коды и коды погашений (Получить коды)
Скачать взломанный APK файл
Скачать ODYSSEY Ionic Solids MOD APK
Запросить взлом