WearLinq Взлом 1.0.4 + Чит Коды
Разработчик: WearLinq, Inc.
Категория: Медицина
Цена: Бесплатно
Версия: 1.0.4
ID: com.wearlinq.ios.prod
Скриншоты
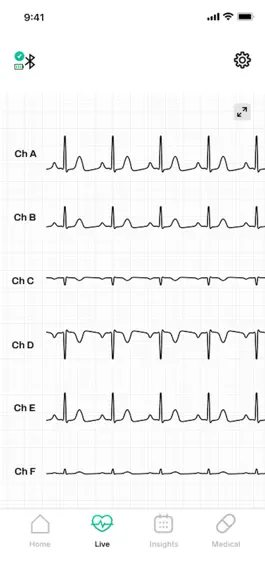
Описание
Welcome to WearLinq!
RECORD YOUR EKG AND CONNECT TO A CARDIOLOGIST
Record you EKG data 24/7 with the FDA-cleared eWave monitor and review your report with a board-certified cardiologist over video.
MEET THE EWAVE
- 24/7 continuous multi-channel EKG recording wherever you are
- Direct transmission and display in the WearLinq app
- Keep the eWave after your prescription period to capture futher symptoms
HOW DOES IT WORK?
1. Download the WearLinq app
2. Wear the eWave during your prescription time
3. Connect with a board-certified cardiologist to review your results
NOTE: Requires the eWave device to record your EKG. Neither the app nor the eWave automatically call emergency services or provide treatment.
FDA 510(k) number: K214073 - only cleared for US use.
RECORD YOUR EKG AND CONNECT TO A CARDIOLOGIST
Record you EKG data 24/7 with the FDA-cleared eWave monitor and review your report with a board-certified cardiologist over video.
MEET THE EWAVE
- 24/7 continuous multi-channel EKG recording wherever you are
- Direct transmission and display in the WearLinq app
- Keep the eWave after your prescription period to capture futher symptoms
HOW DOES IT WORK?
1. Download the WearLinq app
2. Wear the eWave during your prescription time
3. Connect with a board-certified cardiologist to review your results
NOTE: Requires the eWave device to record your EKG. Neither the app nor the eWave automatically call emergency services or provide treatment.
FDA 510(k) number: K214073 - only cleared for US use.
История обновлений
1.0.4
2023-03-28
Bugfixes.
1.0.3
2023-02-28
Bug fixes and minor improvements.
1.0.2
2023-02-08
Minor Bug Fixes.
1.0.1
2022-12-04
Updated App Store name.
1.0
2022-11-26
Способы взлома WearLinq
- Промо коды и коды погашений (Получить коды)
Скачать взломанный APK файл
Скачать WearLinq MOD APK
Запросить взлом