EndeavorRx Insight® Hack 2.3.0 + Redeem Codes
Companion app for parents
Developer: Akili Interactive Labs, Inc.
Category: Medical
Price: Free
Version: 2.3.0
ID: com.akiliinteractive.c01
Screenshots
Description
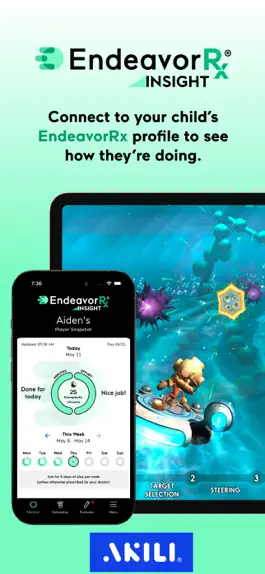
Follow your child's in-game treatment journey with EndeavorRx Insight: the official EndeavorRx® companion app for parents.
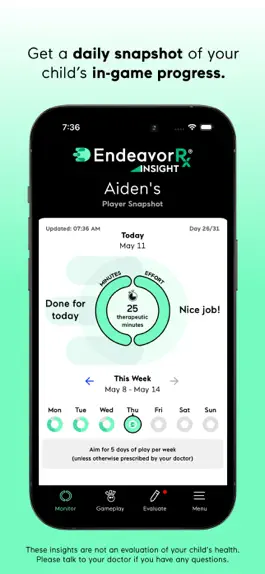
This free app connects directly to your child’s EndeavorRx treatment, so you can get a daily snapshot of your child's in-game mission completion and effort score.
KEY FEATURES:
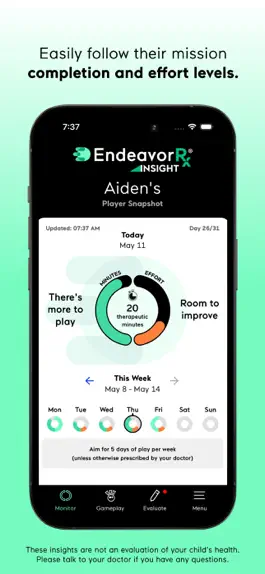
- VIEW MISSION COMPLETION — See if your child's completed all of their play for the day, so you can help them stay on track.
- MONITOR DAILY EFFORT — By analyzing in-game interactions, we calculate a daily score to give you a sense of your child's level of effort and if your child is playing correctly according to the rules.
- TRACK SYMPTOMS — By using the 21 question caregiver assessment.
- EXPORT REPORTS — Generate reports and share the results with your child’s health care provider to inform the conversation and treatment planning.
- GET SUPPORT — Having an issue? The app can help connect you to our Akili Assist® support team.
Important: In order to use EndeavorRx Insight, your child must already have an active prescription for the EndeavorRx digital treatment and be connected to the internet.
WHAT IS ENDEAVORRX?
EndeavorRx is the first & only FDA-authorized video game treatment for inattention for kids 8-12 with ADHD. Access to treatment in the game requires a prescription from your child's healthcare provider.
Created by world-class neuroscientists and award-winning game designers, EndeavorRx is powered by Selective Stimulus Management Engine (SSME™) technology that is designed to challenge and target areas of the brain that play a key role in attention function.
For more information, and to start a virtual appointment with a doctor or get tips on how to talk to your current doctor to determine if EndeavorRx may be right for your child, visit EndeavorRx.com.
INDICATIONS FOR USE
EndeavorRx is a digital therapeutic indicated to improve attention function as measured by computer-based testing in children ages 8-12 years old with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue. Patients who engage with EndeavorRx demonstrate improvements in a digitally assessed measure, Test of Variables of Attention (TOVA®), of sustained and selective attention and may not display benefits in typical behavioral symptoms, such as hyperactivity. EndeavorRx should be considered for use as part of a therapeutic program that may include clinician-directed therapy, medication, and/or educational programs, which further address symptoms of the disorder.
SIDE EFFECTS
No serious adverse events were reported. Of 538 participants in trials supporting EndeavorRx authorization, 50 participants (9.3%) experienced treatment-related adverse events (probable, likely), and three participants experienced treatment-related adverse events with the digital control, in studies where a control was used. Associated adverse events included frustration (6.1%), headache (1.3%), dizziness (0.6%), emotional reaction (0.4%), nausea (0.4%), and aggression (0.2%). All adverse events were generally transient. Only 3 events led to device discontinuation, and no subject reported lasting or irreversible effects after discontinuation.
Akili, EndeavorRx, Endeavor, Akili Care, ADHD Insight, Insight, EndeavorRx Insight, SSME, Play Your Medicine, and Akili Assist, as well as the logos for each, are trademarks or registered trademarks of Akili Interactive Labs, Inc. Other trademarks are trademarks or registered trademarks of their respective owners. Please see the Terms of Use below for applicable patent protection for EndeavorRx.
Terms of Use: https://my.akili.care/terms
Privacy Notice: https://my.akili.care/privacy
This free app connects directly to your child’s EndeavorRx treatment, so you can get a daily snapshot of your child's in-game mission completion and effort score.
KEY FEATURES:
- VIEW MISSION COMPLETION — See if your child's completed all of their play for the day, so you can help them stay on track.
- MONITOR DAILY EFFORT — By analyzing in-game interactions, we calculate a daily score to give you a sense of your child's level of effort and if your child is playing correctly according to the rules.
- TRACK SYMPTOMS — By using the 21 question caregiver assessment.
- EXPORT REPORTS — Generate reports and share the results with your child’s health care provider to inform the conversation and treatment planning.
- GET SUPPORT — Having an issue? The app can help connect you to our Akili Assist® support team.
Important: In order to use EndeavorRx Insight, your child must already have an active prescription for the EndeavorRx digital treatment and be connected to the internet.
WHAT IS ENDEAVORRX?
EndeavorRx is the first & only FDA-authorized video game treatment for inattention for kids 8-12 with ADHD. Access to treatment in the game requires a prescription from your child's healthcare provider.
Created by world-class neuroscientists and award-winning game designers, EndeavorRx is powered by Selective Stimulus Management Engine (SSME™) technology that is designed to challenge and target areas of the brain that play a key role in attention function.
For more information, and to start a virtual appointment with a doctor or get tips on how to talk to your current doctor to determine if EndeavorRx may be right for your child, visit EndeavorRx.com.
INDICATIONS FOR USE
EndeavorRx is a digital therapeutic indicated to improve attention function as measured by computer-based testing in children ages 8-12 years old with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue. Patients who engage with EndeavorRx demonstrate improvements in a digitally assessed measure, Test of Variables of Attention (TOVA®), of sustained and selective attention and may not display benefits in typical behavioral symptoms, such as hyperactivity. EndeavorRx should be considered for use as part of a therapeutic program that may include clinician-directed therapy, medication, and/or educational programs, which further address symptoms of the disorder.
SIDE EFFECTS
No serious adverse events were reported. Of 538 participants in trials supporting EndeavorRx authorization, 50 participants (9.3%) experienced treatment-related adverse events (probable, likely), and three participants experienced treatment-related adverse events with the digital control, in studies where a control was used. Associated adverse events included frustration (6.1%), headache (1.3%), dizziness (0.6%), emotional reaction (0.4%), nausea (0.4%), and aggression (0.2%). All adverse events were generally transient. Only 3 events led to device discontinuation, and no subject reported lasting or irreversible effects after discontinuation.
Akili, EndeavorRx, Endeavor, Akili Care, ADHD Insight, Insight, EndeavorRx Insight, SSME, Play Your Medicine, and Akili Assist, as well as the logos for each, are trademarks or registered trademarks of Akili Interactive Labs, Inc. Other trademarks are trademarks or registered trademarks of their respective owners. Please see the Terms of Use below for applicable patent protection for EndeavorRx.
Terms of Use: https://my.akili.care/terms
Privacy Notice: https://my.akili.care/privacy
Version history
2.3.0
2023-06-29
EndeavorRx® patient progress reports are now available in the app. Complete the clinically-validated assessments and share the results with your child’s health care provider to inform the conversation and treatment planning.
2.2.2
2023-05-18
This release includes an update to your child's reported EndeavorRx daily play time. Now, in EndeavorRx Insight, you'll see actual therapeutic minutes (also called Mission Minutes) played, rather than the Fuel-based limit that was previously in place.
2.2.1
2023-04-27
We've made some minor enhancements, improvements, and bug fixes.
-----------
The Vanderbilt Assessment is now in EndeavorRx Insight®. Take the assessment to help you better understand your child's symptoms as they use EndeavorRx®. Share that data with your child's licensed health care provider to inform treatment planning.
-----------
The Vanderbilt Assessment is now in EndeavorRx Insight®. Take the assessment to help you better understand your child's symptoms as they use EndeavorRx®. Share that data with your child's licensed health care provider to inform treatment planning.
2.2.0
2023-04-20
The Vanderbilt Assessment is now in EndeavorRx Insight®. Take the assessment to help you better understand your child's symptoms as they use EndeavorRx®. Share that data with your child's licensed health care provider to inform treatment planning.
2.1.1
2023-02-21
We've made some minor enhancements, improvements, and bug fixes.
2.1.0
2022-12-05
We've added a new Gameplay tab that lets caregviers follow along with their patients EndeavorRx journey.
Caregivers can now view fun and useful gameplay info including:
- Total therapeutic minutes played
- Number of game worlds completed
- Number of mystic gems unlocked
We hope you'll use this new feature to follow your patient's journey and encourage them to have the best treatment possible!
Caregivers can now view fun and useful gameplay info including:
- Total therapeutic minutes played
- Number of game worlds completed
- Number of mystic gems unlocked
We hope you'll use this new feature to follow your patient's journey and encourage them to have the best treatment possible!
2.0.0
2022-10-19
Caregivers can now pull-to-refresh to see their patient's latest gameplay data.
We've improved error messaging so errors are easier to understand and resolve.
We've improved error messaging so errors are easier to understand and resolve.
v1.2.1
2022-06-16
We've made some minor enhancements, improvements, and bug fixes.
1.2.0
2022-06-13
We added support for multiple treatments in this release, along with enhancements, improvements and bug fixes.
1.1.0
2022-04-07
We've made some minor enhancements, improvements, and bug fixes.
Users can now initiate account deletion from the app menu.
Users can now initiate account deletion from the app menu.
1.0.1
2022-01-24
We've made some minor enhancements, improvements, and bug fixes.
1.0
2021-12-13
Ways to hack EndeavorRx Insight®
- Redeem codes (Get the Redeem codes)
Download hacked APK
Download EndeavorRx Insight® MOD APK
Request a Hack
Ratings
4.4 out of 5
105 Ratings
Reviews
fhdyx hchfdgdsjk,
Endeavor RX is the best thing for me
Hi there this is Haylen I just think you are going to be famous one day this has helped thousands of people with ADHD 🐸🐻🦎🦄🤑
kjp8899,
Log on doesn't work
Not sure why they ask you to create an account to monitor your child - thought I had the wrong password because I kept receiving error message- to find that when I tried to change my password , I received the 'you can't use the same password' message.
Changed to new password tried to log in and received same error message that 'link is expired' quite embarrassing for a 'tech' app.
Glad I only signed up for a month without auto renewal...
Changed to new password tried to log in and received same error message that 'link is expired' quite embarrassing for a 'tech' app.
Glad I only signed up for a month without auto renewal...
Mommy of Sad Boy,
Useless
There is barely any information at all. I already know that my son completes his time because I monitor him while he is playing to make sure he completes all his missions for the day. When he completes a mission it shows his longest focus streak, how long it took to complete the mission, etc. of course he has no interest in this information and clicks right past it. That information would be so useful on the parent side so we could actually see the data that goes with what our child is completing.
Hoopjrulz,
Gives you zero metrics
Would be more useful to actually see trends over time or where my child is struggling and what improvements are being made. All this tells me is how many minutes and effort. I have no idea what effort means in real life. Maybe since this is only our first week it’ll tell me more in the future? Not particularly useful. Also, please put a timer in the app maybe something the parent can set. Apparently we aren’t getting enough game time in. I have to set my own timer.
app is good said me,
Bad Timer
Power doesn’t work it says I’ve only been going for four minutes on the timer in my house says that I’ve been doing it for 16 minutes it’s just bad can you please help it literally is so annoying I just use my own time right now