ODYSSEY Basic Crystal Lattices Hack 2.0.3 + Redeem Codes
Developer: Wavefunction, Inc
Category: Education
Price: $3.99 (Download for free)
Version: 2.0.3
ID: com.wavefun.OdyCrystalLattices
Screenshots
Description
What are the basic lattice types encountered in the structures of elements and compounds? Is there more than one way to efficiently pack simple spheres? Why are copper and many other metals so malleable?
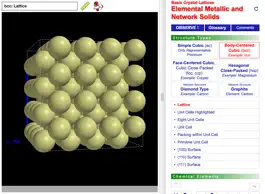
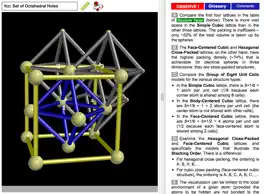
ODYSSEY Basic Crystal Lattices helps introduce the most important lattice types of elementary crystallography. Generic models are provided for the simple cubic lattice, body-centered cubic lattice, face-centered cubic lattice, hexagonally close-packed lattice, diamond lattice, and graphite lattice. Conventional and primitive unit cells are shown, coordination numbers are highlighted, and tetrahedral and octahedral holes are visualized. In total more than 160 models are available, including a comprehensive set of models for all those elements that crystallize in one of the listed structure types.
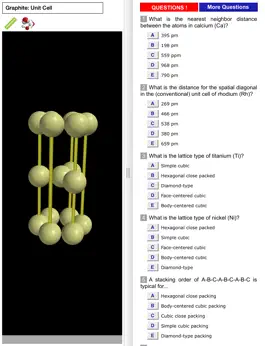
Models can be inspected at any orientation and zoom level and with a choice of drawing radius for the atoms. For most models, a “clipping sphere” can be defined, highlighting the local surroundings of a selected atom and hiding the remaining structure. The distances between atoms can be measured. A glossary, comments section, and set of multiple choice questions (with randomized options) are also included.
In almost no subject is three-dimensional visualization more important than in crystallography. ODYSSEY Basic Crystal Lattices complements and clarifies textbook descriptions of some core crystallographic concepts.
ODYSSEY Basic Crystal Lattices helps introduce the most important lattice types of elementary crystallography. Generic models are provided for the simple cubic lattice, body-centered cubic lattice, face-centered cubic lattice, hexagonally close-packed lattice, diamond lattice, and graphite lattice. Conventional and primitive unit cells are shown, coordination numbers are highlighted, and tetrahedral and octahedral holes are visualized. In total more than 160 models are available, including a comprehensive set of models for all those elements that crystallize in one of the listed structure types.
Models can be inspected at any orientation and zoom level and with a choice of drawing radius for the atoms. For most models, a “clipping sphere” can be defined, highlighting the local surroundings of a selected atom and hiding the remaining structure. The distances between atoms can be measured. A glossary, comments section, and set of multiple choice questions (with randomized options) are also included.
In almost no subject is three-dimensional visualization more important than in crystallography. ODYSSEY Basic Crystal Lattices complements and clarifies textbook descriptions of some core crystallographic concepts.
Version history
2.0.3
2022-08-01
iOS 15 fixes- minor content updates
2.0.2
2018-12-21
Compatibility fix for certain devices
2.0.1
2016-01-21
- iPad Pro compatibility
- Quarter point penalty for incorrect answers removed
- Quarter point penalty for incorrect answers removed
2.0.0
2015-08-27
- Consistent naming and ordering of submodels across structure types
- A few low-index surfaces for metallic structure types
- Unit cells available for all included elements
- Room temperature lattice vibrations for many elements
- Number of questions almost doubled
- App loads faster
- Bug fixes
- A few low-index surfaces for metallic structure types
- Unit cells available for all included elements
- Room temperature lattice vibrations for many elements
- Number of questions almost doubled
- App loads faster
- Bug fixes
1.0.1
2014-12-02
- Touch interface atom picking improved
- Model and text clarifications
- Bug fixes
- Model and text clarifications
- Bug fixes
1.0.0
2014-07-26
Ways to hack ODYSSEY Basic Crystal Lattices
- Redeem codes (Get the Redeem codes)
Download hacked APK
Download ODYSSEY Basic Crystal Lattices MOD APK
Request a Hack