SAR Table Hack 1.3.11 + Redeem Codes
Developer: Molecular Materials Informatics, Inc.
Category: Productivity
Price: Free
Version: 1.3.11
ID: com.molmatinf.sartable
Screenshots
Description
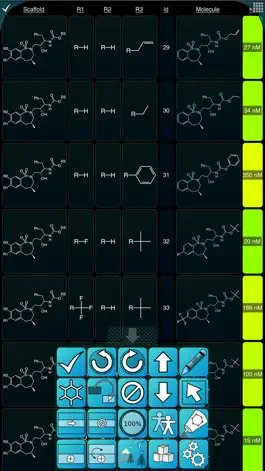
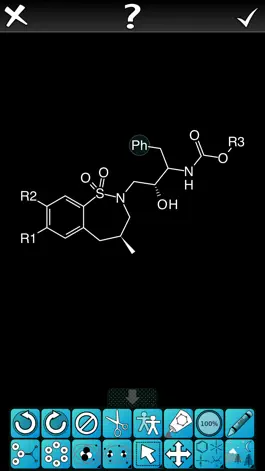
The SAR Table app is designed for creating tables containing a series of related structures, their activity/property data, and associated text. Structures are represented by scaffolds and substituents, which are combined together to automatically generate a construct molecule. The table editor has many convenience features and data checking cues to make the data entry process as efficient as possible.
Data can be shared, and used as the basis for creating presentation quality output.
Data can be shared, and used as the basis for creating presentation quality output.
Version history
1.3.11
2018-02-06
This app has been updated by Apple to display the Apple Watch app icon.
Maintenance release.
Maintenance release.
1.3.10
2017-11-13
Updated for iOS 11.
1.3.9
2015-09-12
Maintenance release.
1.3.8
2015-02-03
Importing and exporting of SAR Table datasheets is now possible using app intensions: files can be uploaded or downloaded from online file sharing services like iCloud and Dropbox.
1.3.7
2014-11-15
Updated for iOS 8 and new iPhone 6 form factors. Also improved integration of tool tips within the molecular diagram sketcher.
1.3.6
2014-03-13
New web-supported feature to "homogenise": any two fragments with the same canonical representation will be assigned the same sketch. Also several bugfixes.
1.3.5
2013-10-08
Updated for iOS 7, and numerous usability improvements.
1.3.4
2013-03-20
It is now possible to match multiple scaffolds in a single request: select multiple rows (using the new selection menu) and submit the match request. Any molecules that can be matched unambiguously to an available scaffold will be assigned automagically.
Also, missing values that are predicted using a model are now displayed numerically, and are also included when exporting data.
Also, missing values that are predicted using a model are now displayed numerically, and are also included when exporting data.
1.3.3
2013-03-08
From the matrix view, it is possible to use a previously constructed model to estimate properties for each cell: the webservice creates a sampling of hypothetical compounds, and returns an average of predicted values for the ensemble.
Looking up structure fragments when editing cells displays an activity distribution for each of the fragments (e.g. scaffold, R1, R2, etc.) to assist in selection. Also, a number of minor bug fixes and ergonomic improvements
Looking up structure fragments when editing cells displays an activity distribution for each of the fragments (e.g. scaffold, R1, R2, etc.) to assist in selection. Also, a number of minor bug fixes and ergonomic improvements
1.3.2
2013-02-16
The "matrix view" is now interactive: tap on a cell to examine the contents, and create virtual compounds.
Scaffolds can now be searched for in public databases (PubChem, ChEBI): template matching algorithms are used to obtain compounds that are compatible with the series, and assign them for addition as new rows.
Scaffolds can now be searched for in public databases (PubChem, ChEBI): template matching algorithms are used to obtain compounds that are compatible with the series, and assign them for addition as new rows.
1.3.1
2013-01-31
Properties can be calculated using a webservice, including molecular weight/formula, log P, molar refractivity and topological polar surface area.
1.3
2013-01-05
Model building: can invoke a webservice to build a structure-property model using current data, then use the model to predict values for structures with unknown properties.
Also, modified to work with the iPhone 5 form factor.
Also, modified to work with the iPhone 5 form factor.
1.2.2
2012-06-25
Home page now uses menu icons instead of text. Matrix view adds a "fit" button to toggle between natural size vs. all-on-screen.
1.2.1
2012-06-05
In the matrix view, rows and columns can be sorted. Also, SAR Tables can be shared via Twitter.
1.2
2012-05-16
Data can now be displayed using the Matrix View: two fields are plotted against each other (e.g. R1 vs R2), and one or more responses (numeric properties) are displayed at the points of intersection. Colour schemes are used to denote intensity.
1.1.4
2012-05-01
Property fields can be associated with Schemes, which specify default units, range, and colouring, which allows the display to use colour-coding to highlight the values.
Property fields also now have a standard error component.
Also, significant performance improvements for retina-class devices.
Property fields also now have a standard error component.
Also, significant performance improvements for retina-class devices.
1.1.3
2012-04-13
Scalar fields (id, text, property) can be changed to different types, which is especially helpful for imported data.
Renaming substituent fields (e.g. R1 to R2) can auto-update all scaffolds and substituents to reflect the change.
Also, the chemical structure editor has been updated to make it more user friendly.
Renaming substituent fields (e.g. R1 to R2) can auto-update all scaffolds and substituents to reflect the change.
Also, the chemical structure editor has been updated to make it more user friendly.
1.1.2
2012-03-13
Scaffolds can be "re-matched": a convenient way to select a different substituent configuration when the scaffold is symmetrical or degenerate.
Also, improved exporting possibilities: easy creation of SDfiles and molecular datasheets.
Also, improved exporting possibilities: easy creation of SDfiles and molecular datasheets.
1.1.1
2012-02-15
Maintenance release: minor improvements and bugfixes.
1.1
2012-01-10
Scaffold matching feature now available: uses a substructure search algorithm to match a scaffold core within a whole molecule, and derive the substituent labels, and the substituent fragments automatically. When combined with important molecules, this is an invaluable shortcut for classifying structures based on scaffold/substituent combinations.
1.0.2
2011-11-21
A number of user interface improvements, such as duplicate row, and the ability to apply edits to multiple cells. Auto-generated structures now apply a localised depiction technique to obtain nicer results. Tables can be exported as Microsoft Excel documents.
1.0.1
2011-11-16
SAR Tables can be exported as Microsoft Word documents with embedded vector graphics, or as HTML files with embedded SVG.
1.0
2011-11-07
Ways to hack SAR Table
- Redeem codes (Get the Redeem codes)
Download hacked APK
Download SAR Table MOD APK
Request a Hack
Ratings
5 out of 5
1 Ratings